Menu
PHARMA
HOME > MARKETS & APPLICATIONS > PHARMA
PHARMA
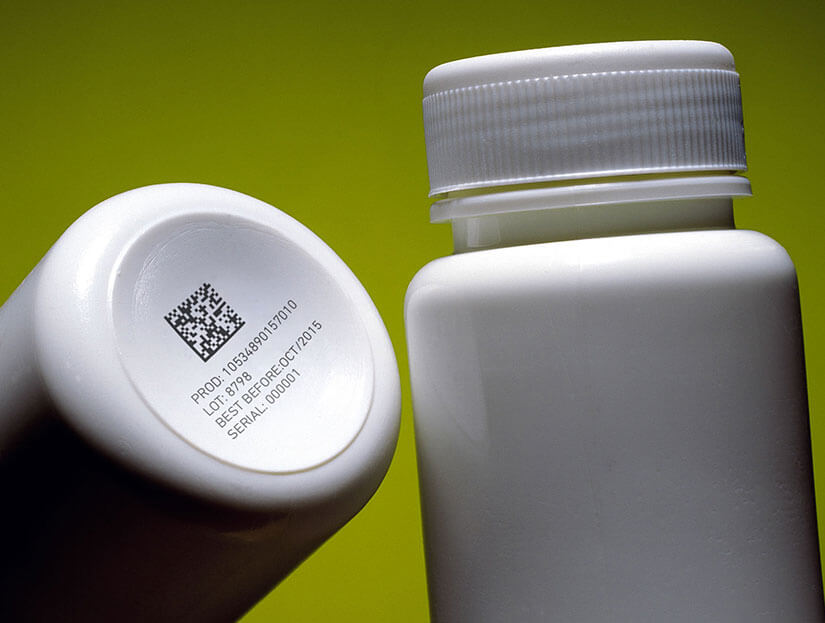
In the pharmaceutical sector, identification and traceability of medicines are of vital importance
Pharmaceutical product manufacturers must ensure that medicinal products have a unique serial number, as well as coding the packaging and even the tablets according to current legislation, taking special care that the marking is legible, of high quality and will last throughout the medicine’s lifetime.
Coding and marking of pharmaceutical products must guarantee the identification of unsafe products by means of traceability. Apart from facilitating their speedy withdrawal from the market, if necessary, they must also help in the fight against counterfeit medicines, given the enormous consequences that these can cause to people’s health.
With Bradma Macsa ID solutions coding can be carried out on a wide variety of materials, like monocartons, paper, cardboard, glass, and plastic, as well as on the product itself, pills or tablets, their blister packs, cases, medicine boxes and pallets.




----------
VIDEOS
----------

CONTACT US
Receive more information about food traceability

MORE
MARKET & APPICATIONS
With Bradma Macsa ID technologies you will be able to mark a great variety of materials and substrates, belonging to various markets, and code them meeting the requirement of product traceability.




